
Cross Metathesis
![]()
The Transalkylidenation Of Two Terminal Alkenes Under Release Of Ethene, Catalyzed By Ruthenium Carbenoids (Grubbs Catalyst). Statistically, The Reaction Can Lead To Three Possible Pairs Of Geometric Isomers, I.e. E/Z pairs For Two Homocouplings And The Cross-coupling (R-CH=CH-R, R'-CH=CH-R', And R-CH=CH-R') - A Total Of 6 Products.
The Selectivity Of This Reaction Is Currently Undergoing Further Study, But Various Examples Exist In Which Two Alkenes With Different Reactivity Give The Cross-coupled Product With Excellent Yields And Excellent Selectivity.
Recent Literature

Rate Enhanced Olefin Cross-Metathesis Reactions: The Copper Iodide Effect
K. Voigtritter, S. Ghorai, B. H. Lipshutz, J. Org. Chem., 2011, 76, 4697-4702.

Olefin Cross-Metathesis Reactions At Room Temperature Using The Nonionic Amphiphile “PTS”: Just Add Water
B. H. Lipshutz, G. T. Aquinaldo, S. Ghorai, K. Voigtritter, Org. Lett., 2008, 10, 1325-1328.

Increased Efficiency In Cross-Metathesis Reactions Of Sterically Hindered Olefins
I. C. Stewart, C. J. Douglas, R. H. Grubbs, Org. Lett., 2008, 10, 441-444.

Stereoretentive Olefin Metathesis Made Easy: In Situ Generation Of Highly Selective Ruthenium Catalysts From Commercial Starting Materials
D. S. Müller, I. Curbet, Y. Raoul, J. Le Nôtre, O. Baslé, M. Mauduit, Org. Lett., 2018, 20, 6822-6826.

Synthesis Of Z- Or E-Trisubstituted Allylic Alcohols And Ethers By Kinetically Controlled Cross-Metathesis With A Ru Catechothiolate Complex
C. Xu, Z. Liu, S. Torker, X. Shen, D. Xu, A. H. Hoveyda, J. Am. Chem. Soc., 2017, 139, 15640-15643.
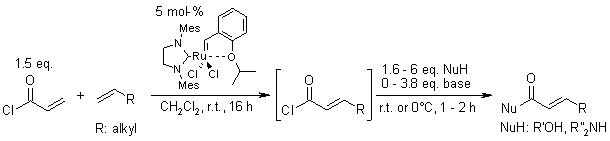
Acryloyl Chloride: An Excellent Substrate For Cross-Metathesis. A One-Pot Sequence For The Synthesis Of Substituted α,β-Unsaturated Carbonyl Derivatives
L. Ferrié, S. Bouzbouz, J. Cossy, Org. Lett., 2009, 11, 5446-5448.

A Rapid And Simple Cleanup Procedure For Metathesis Reactions
B. R. Galan, K. P. Kalbarczyk, S. Szczepankiewicz, J. B. Keister, S. T. Diver, Org. Lett., 2007, 9, 1203-1206.

Direct Synthesis Of Z-alkenyl Halides Through Catalytic Cross-metathesis
M. J. Koh, T. T. Ngyuen, H. Zhang, R. R. Schrock, A. H. Hoveyda, Nature, 2016, 531, 459-465.

Regioselective Cross-Metathesis Reaction Induced By Steric Hindrance
S. BouzBouz, R. Simmons, J. Cossy, Org. Lett., 2004, 6, 3465-3467.

An Efficient Synthesis Of Nitroalkenes By Alkene Cross Metathesis: Facile Access To Small Ring Systems
G. P. Marsh, P. J. Parsons, C. McCarthy, X. G. Corniquet, Org. Lett., 2007, 9, 2613-2616.

Selective Synthesis Of (2Z,4E)-Dienyl Esters By Ene-Diene Cross Metathesis
G. Moura-Letts, D. P. Curran, Org. Lett., 2007, 9, 5-8.

Advanced Fine-Tuning Of Grubbs/Hoveyda Olefin Metathesis Catalysts: A Further Step Toward An Optimum Balance Between Antinomic Properties
M. Bieniek, R. Bujok, M. Cabaj, N. Lugan, G. Lavigne, D. Arlt, K. Grela, J. Am. Chem. Soc., 2006, 128, 13652-13653.

A Good Bargain: An Inexpensive, Air-Stable Ruthenium Metathesis Catalyst Derived From α-Asarone
K. Grela, M. Kim, Eur. J. Org. Chem., 2003, 963-966.

The Facile Preparation Of Alkenyl Metathesis Synthons
T. W. Baughman, J. C. Sworen, K. B. Wagener, Tetrahedron, 2004, 60, 10943-10948.

Ruthenium-Catalyzed Tandem Olefin Metathesis-Oxidations
A. A. Scholte, M. H. An, M. L. Snapper, Org. Lett., 2006, 8, 4759-4762.

Ruthenium-Catalyzed Tandem Cross-Metathesis/Wittig Olefination: Generation Of Conjugated Dienoic Esters From Terminal Olefins
R. P. Murelli, M. L. Snapper, Org. Lett., 2007, 9, 1749-1752.

Preparation Of Aliphatic Ketones Through A Ruthenium-Catalyzed Tandem Cross-Metathesis/Allylic Alcohol Isomerization
D. Finnegan, B. A. Seigal, M. L. Snapper, Org. Lett., 2006, 8, 2603-2606.

Heteroaromatic Synthesis Via Olefin Cross-Metathesis: Entry To Polysubstituted Pyridines
T. J. Donohoe, J. A. Basutto, J. F. Bower, A. Rathi, Org. Lett., 2011, 13, 1036-1039.

Cross Metathesis Of N-Allylamines And α,β-Unsaturated Carbonyl Compounds: A One-Pot Synthesis Of Substituted Pyrroles
S. Shafi, M. K?dziorek, K. Grela, Synlett, 2011, 124-128.