
Heck Reaction
Heck Reaction
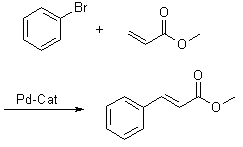
The Palladium-catalyzed C-C Coupling Between Aryl Halides Or Vinyl Halides And Activated Alkenes In The Presence Of A Base Is Referred As The "Heck Reaction". Recent Developments In The Catalysts And Reaction Conditions Have Resulted In A Much Broader Range Of Donors And Acceptors Being Amenable To The Heck Reaction.
One Of The Benefits Of The Heck Reaction Is Its Outstanding trans selectivity.
Mechanism Of The Heck Reaction

Recent Literature

Trifunctional N,N,O-terdentate Amido/pyridyl Carboxylate Pd(II) Complexes Were Highly Active And Stable Phosphine-free Catalysts For Heck And Room-temperature Suzuki Reactions With High Turnover Numbers.
M. L. Kantam, P. Srinivas, J. Yadav, P. R. Likhar, S. Bhargava, J. Org. Chem., 2009, 74, 4882-4885.

New N-Heterocyclic Carbene Palladium Complex/Ionic Liquid Matrix Immobilized On Silica: Application As Recoverable Catalyst For The Heck Reaction
B. Karimi, D. Enders, Org. Lett., 2006, 8, 1237-1240.

Pd(quinoline-8-carboxylate)2 as A Low-Priced, Phosphine-Free Catalyst For Heck And Suzuki Reactions
X. Cui, J. Li, Z.-P. Zhang, Y. Fu, L. Liu, Q.-X. Guo, J. Org. Chem., 2007, 72, 9342-9345.

1,1'-Methylene-3,3'-bis[(N-(tert-butyl)imidazol-2-ylidene] And Its Effect In Palladium-Catalyzed C-C Coupling
S. Nadri, E. Rafiee, S. Jamali, M. Joshaghani, Synlett, 2015, 26, 619-624.

Heck Reactions Catalyzed By Ultrasmall And Uniform Pd Nanoparticles Supported On Polyaniline
L. Yu, Y. Huang, Z. Wei, Y. Ding, C. Su, Q. Xu, J. Org. Chem., 2015, 80, 8677-8683.

Triethanolamine As An Efficient And Reusable Base, Ligand And Reaction Medium For Phosphane-Free Palladium-Catalyzed Heck Reactions
H. J. Li, L. Wang, Eur. J. Org. Chem., 2006, 5101-5102.

Triaryl Phosphine-functionalized N-heterocyclic Carbene Ligands For Heck Reaction
A.-E. Wang, J.-H. Xie, L.-X. Wang, Q.-L. Zhou, Tetrahedron, 2005, 61, 259-266.

An Efficient And General Method For The Heck And Buchwald-Hartwig Coupling Reactions Of Aryl Chlorides
D.-H. Lee, A. Taher, S. Hossain, M.-J. Jin, Org. Lett., 2011, 13, 5540-5543.

Palladium-Catalyzed Heck Reaction Of Aryl Chlorides Under Mild Conditions Promoted By Organic Ionic Bases
H.-J. Xu, Y.-Q. Zhao, X.-F. Zhou, J. Org. Chem., 2011, 76, 8036-8041.

Irradiation-Induced Heck Reaction Of Unactivated Alkyl Halides At Room Temperature
G.-Z. Wang, R. Shang, W.-M. Cheng, Y. Fu, J. Am. Chem. Soc., 2017, 139, 18307-18312.
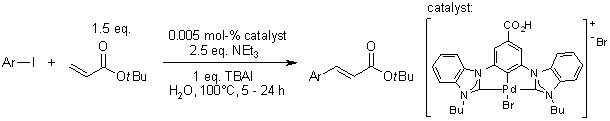
Efficient Aqueous-Phase Heck Reaction Catalyzed By A Robust Hydrophilic Pyridine-Bridged Bisbenzimidazolylidene-Palladium Pincer Complex
Z. Wang, X. Feng, W. Fang, T. Tu, Synlett, 2011, 951-954.

A Palladacycle Phosphine Mono-ylide Complex Is As An Efficient Catalyst For The Mizoroki-Heck Cross-coupling Reaction Of Aromatic Or Aliphatic Olefins With A Broad Range Of Aryl Bromides And Chlorides. The Reactions Proceeded In Good Yields In The Presence Of Low Loadings Of Palladium (10 Ppm) Under Aerobic Conditions. High Catalyst Activities With Turnover Frequencies Of Up To 20,000 H-1 were Observed At 130°C.
S. J. Sabounchi, M. Ahmadi, T. Azizi, M. Panahimehr, Synlett, 2014, 25, 336-342.

An Efficient And Simple Protocol For Phosphine-free Heck Reactions In Water In The Presence Of A Pd(L-proline)2 complex As The Catalyst Under Controlled Microwave Irradiation Conditions Is Versatile And Provides Excellent Yields Of Products In Short Reaction Times. The Reaction System Minimizes Costs, Operational Hazards And Environmental Pollution.
B. K. Allam, K. N. Singh, Synthesis, 2011, 1125-1131.

Heck Couplings At Room Temperature In Nanometer Aqueous Micelles
B. H. Lipshutz, B. R. Taft, Org. Lett., 2008, 10, 1329-1332.

Efficient Aqueous-Phase Heck And Suzuki Couplings Of Aryl Bromides Using Tri(4,6-dimethyl-3- Sulfonatophenyl)phosphine Trisodium Salt (TXPTS)
L. R. Moore, K. H. Shaughnessy, Org. Lett., 2004, 6, 225-228.

Poly(ethylene Glycol) (PEG) As A Reusable Solvent Medium For Organic Synthesis. Application In The Heck Reaction
S. Chandrasekhar, C. Narsihmulu, S. S. Sultana, N. R. Reddy, Org. Lett., 2002, 4, 4399-4401.
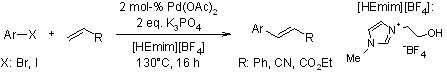
Functionalized Ionic Liquid As An Efficient And Recyclable Reaction Medium For Phosphine-Free Palladium-Catalyzed Heck Reaction
L. Zhou, L. Wang, Synthesis, 2006, 2649-2652.

Brønsted Guanidine Acid-Base Ionic Liquids: Novel Reaction Media For The Palladium-Catalyzed Heck Reaction
S. Li, Y. Lin, H. Xie, S. Zhang, J. Xu, Org. Lett., 2006, 8, 391-394.

Practical Heck-Mizoroki Coupling Protocol For Challenging Substrates Mediated By An N-Heterocyclic Carbene-Ligated Palladacycle
E. A. B. Kantchev, G.-R. Peh, C. Zhang, J. Y. Ying, Org. Lett., 2008, 10, 3949-3952.
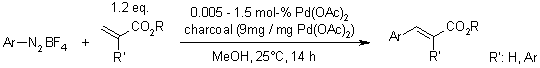
A Sustainable Procedure Combining The Advantages Of Both Homogeneous And Heterogeneous Catalysis For The Heck-Matsuda Reaction
C. Rossy, E. Fouquet, F.-X. Felpin, Synthesis, 2012, 44, 37-41.

Operationally Simple And Highly (E)-Styrenyl-Selective Heck Reactions Of Electronically Nonbiased Olefins
E. W. Werner, M. S. Sigman, J. Am. Chem. Soc., 2011, 133, 9692-9695.

Pd-mBDPP-Catalyzed Regioselective Internal Arylation Of Electron-Rich Olefins By Aryl Halides
S. Liu, N. Berry, N. Thomson, A. Pettman, Z. Hyder, J. Mo, J. Xiao, J. Org. Chem., 2006, 71, 7467-7470.

Regioselective Heck Vinylation Of Electron-Rich Olefins With Vinyl Halides: Is The Neutral Pathway In Operation?
M. McConville, O. Saidi, J. Blacker, J. Xiao, J. Org. Chem., 2009, 74, 2692-2698.

The Heck Reaction Of Electron-Rich Olefins With Regiocontrol By Hydrogen-Bond Donors
J. Mo, J. Xiao, Angew. Chem. Int. Ed., 2006, 45, 4152-4157.

Palladium-Tetraphosphine Complex Catalysed Heck Reaction Of Vinyl Bromides With Alkenes: A Powerful Access To Conjugated Dienes
M. Lemhadri, A. Battace, F. Berthiol, T. Zair, H. Doucet, M. Santelli, Synthesis, 2008, 1142-1152.

Heck Coupling With Nonactivated Alkenyl Tosylates And Phosphates: Examples Of Effective 1,2-Migrations Of The Alkenyl Palladium(II) Intermediates
A. L. Hansen, J.-P. Ebran, M. Ahlquist, P.-O. Norrby, T. Skydstrup, Angew. Chem. Int. Ed., 2006, 45, 3349-3353.

A New Route To The Synthesis Of (E)- And (Z)-2-Alkene-4-ynoates And Nitriles From vic-Diiodo-(E)-alkenes Catalyzed By Pd(0) Nanoparticles In Water
B. C. Ranu, K. Chattopadhyay, Org. Lett., 2007, 9, 2409-2412.

Synthesis Of 2-Vinylic Indoles And Derivatives Via A Pd-Catalyzed Tandem Coupling Reaction
A. Fayol, Y.-Q. Fang, M. Lautens, Org. Lett., 2006, 8, 4203-4206.

Heck Vinylations Using Vinyl Sulfide, Vinyl Sulfoxide, Vinyl Sulfone, Or Vinyl Sulfonate Derivatives And Aryl Bromides Catalyzed By A Palladium Complex Derived From A Tetraphosphine
A. Battace, T. Zair, H. Doucet, M. Santelli, Synthesis, 2006, 3495-3505.

A Diverted Aerobic Heck Reaction Enables Selective 1,3-Diene And 1,3,5-Triene Synthesis Through C-C Bond Scission
N. J. McAlpine, L. Wang, B. P. Carrow, J. Am. Chem. Soc., 2018, 140, 13634-13639.

Direct Acylation Of Aryl Bromides With Aldehydes By Palladium Catalysis
J. Ruan, O. Saidi, J. A. Iggo, J. Xiao, J. Am. Chem. Soc., 2008, 130, 10510-10511.

Preparation Of Vinyl Silyl Ethers And Disiloxanes Via The Silyl-Heck Reaction Of Silyl Ditriflates
S. E. S. Martin, D. A. Watson, J. Am. Chem. Soc., 2013, 135, 13330-13333.

Direct Synthesis Of Alkenyl Boronic Esters From Unfunctionalized Alkenes: A Boryl-Heck Reaction
W. B. Reid, J. J. Spillane, S. B. Krause, D. A. Watson, J. Am. Chem. Soc., 2016, 138, 5539-5542.

Synthesis Of Trisubstituted Alkenyl Boronic Esters From Alkenes Using The Boryl-Heck Reaction
W. B. Reid, D. A. Watson, Org. Lett., 2018, 20, 6822-6826.

Irradiation-Induced Palladium-Catalyzed Decarboxylative Heck Reaction Of Aliphatic N-(Acyloxy)phthalimides At Room Temperature
G.-Z. Wang, R. Shang, Y. Fu, Org. Lett., 2018, 20, 888-891.

Asymmetric Intermolecular Heck Reaction Of Aryl Halides
C. Wu, J. Zhou, J. Am. Chem. Soc., 2014, 136, 650-652.

Enantiospecific Intramolecular Heck Reactions Of Secondary Benzylic Ethers
M. R. Harris, M. O. Konev, E. R. Jarvo, J. Am. Chem. Soc., 2014, 136, 7825-7828.

Palladium-Catalyzed 6-Endo Selective Alkyl-Heck Reactions: Access To 5-Phenyl-1,2,3,6-tetrahydropyridine Derivatives
X. Dong, Y. Han, F. Yan, Q. Liu, P. Wang, K. Chen, Y. Li, Z. Zhao, Y. Dong, H. Liu, Org. Lett., 2016, 18, 3758-3761.