
Sonogashira Coupling
Sonogashira Coupling

This Coupling Of Terminal Alkynes With Aryl Or Vinyl Halides Is Performed With A Palladium Catalyst, A Copper(I) Cocatalyst, And An Amine Base. Typically, The Reaction Requires Anhydrous And Anaerobic Conditions, But Newer Procedures Have Been Developed Where These Restrictions Are Not Important.
Mechanism Of The Sonogashira Coupling
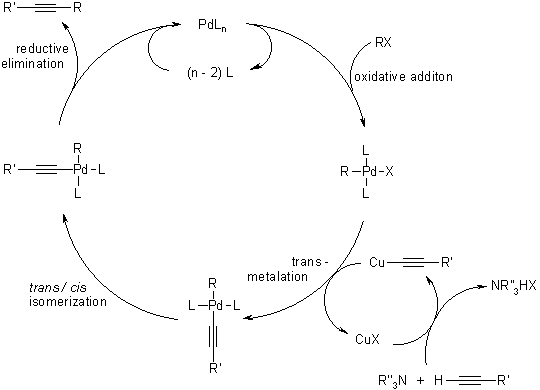
Recent Literature

The First Applications Of Carbene Ligands In Cross-Couplings Of Alkyl Electrophiles: Sonogashira Reactions Of Unactivated Alkyl Bromides And Iodides
M. Eckhardt, G. C. Fu, J. Am. Chem. Soc., 2003, 125, 13642-13643.

Efficient Sonogashira Coupling Reaction Catalyzed By Palladium(II) β-Oxoiminatophosphane Complexes Under Mild Conditions
D.-H. Lee, H. Qiu, M.-H. Cho, I.-M. Lee, M.-J. Jin, Synlett, 2008, 1657-1660.

Hydrazone-Promoted Sonogashira Coupling Reaction With Aryl Bromides At Low Palladium Loadings
T. Mino, S. Suzuki, K. Hirai, M. Sakamoto, T. Fujita, Synlett, 2011, 1277-1280.

Sonogashira Couplings Catalyzed By Collaborative (N-Heterocyclic Carbene)-Copper And -Palladium Complexes
C. W. D. Gallop, M.-T. Chen, O. Navarro, Org. Lett., 2014, 16, 3724-3727.

Sustainable HandaPhos-ppm Palladium Technology For Copper-Free Sonogashira Couplings In Water Under Mild Conditions
S. Handa, J. D. Smith, Y. Zhang, B. S. Takale, F. Gallou, B. H. Lipshutz, Org. Lett., 2018, 20, 542-545.

Designing Homogeneous Copper-Free Sonogashira Reaction Through A Prism Of Pd-Pd Transmetalation
B. A. Martek, M. Gazvoda, D. Urankar, J. Košmrlj, Org. Lett., 2020, 22, 4938-4943.

Pd(PhCN)2Cl2/P(t-Bu)3: A Versatile Catalyst For Sonogashira Reactions Of Aryl Bromides At Room Temperature
T. Hundertmark, A. F. Littke, S. L. Buchwald, G. C. Fu, Org. Lett., 2000, 2, 1729-1731.

Dimethylisosorbide (DMI) As A Bio-Derived Solvent For Pd-Catalyzed Cross-Coupling Reactions
K. L. Wilson, J. Murray, H. F. Sneddon, C. Jamieson, A. J. B. Watson, Synlett, 2018, 29, 2293-2297.

Gold And Palladium Combined For The Sonogashira Coupling Of Aryl And Heteroaryl Halides
B. Panda, T. K. Sarkar, Synthesis, 2013, 45, 817-829.

Modified Palladium-Catalyzed Sonogashira Cross-Coupling Reactions Under Copper-, Amine-, And Solvent-Free Conditions
Y. Liang, Y.-X. Xie, J.-H. Li, J. Org. Chem., 2006, 71, 379-381.

Application Of Recoverable Nanosized Palladium(0) Catalyst In Sonogashira Reaction
P. Li, L. Wang, H. Li, Tetrahedron, 2005, 61, 8633-8640.

Sonogashira Couplings Of Aryl Bromides: Room Temperature, Water Only, No Copper
B. H. Lipshutz, D. W. Chung, B. Rich, Org. Lett., 2008, 10, 3793-3796.

Copper- And Ligand-Free Sonogashira Reaction Catalyzed By Pd(0) Nanoparticles At Ambient Conditions Under Ultrasound Irradiation
A. R. Gholap, K. Venkatesan, R. Pasricha, T. Daniel, R. J. Lahoti, K. V. Srinivasan, J. Org. Chem., 2005, 70, 4869-4872.

CsF-Mediated In Situ Desilylation Of TMS-Alkynes For Sonogashira Reaction
J. S. Capani Jr., J. E. Chochran, J. Liang, J. Org. Chem., 2019, 84, 9378-9384.

Palladacycle-Catalyzed Deacetonative Sonogashira Coupling Of Aryl Propargyl Alcohols With Aryl Chlorides
H. Hu, F. Yang, Y. Wu, J. Org. Chem., 2013, 78, 10506-10507.

Efficient Copper-Free PdCl2(PCy3)2-Catalyzed Sonogashira Coupling Of Aryl Chlorides With Terminal Alkynes
C. Yi, R. Hua, J. Org. Chem., 2006, 71, 2535-2537.

Efficient Palladium-Catalyzed Coupling Of Aryl Chlorides And Tosylates With Terminal Alkynes: Use Of A Copper Cocatalyst Inhibits The Reaction
D. Gelman, S. L. Buchwald, Angew. Chem. Int. Ed., 2003, 42, 5993-5996.

Rapid And Efficient Pd-Catalyzed Sonogashira Coupling Of Aryl Chlorides
H. Huang, H. Liu, H. Jiang, K. Chen, J. Org. Chem., 2008, 73, 6037-6040.

A Highly Efficient Pd-catalyzed Sonogashira Coupling Of Terminal Alkynes With Both Unreactive Electron-rich Fluoroarenes And Electron-poor Fluoroarenes Afforded The Corresponding Internal Alkynes In Good Yields In The Presence Of LiHMDS.
J. He, K. Yang, J. Zhao, S. Cao, Org. Lett., 2019, 21, 9714-9718.

Copper-Free Sonogashira Coupling Reaction With PdCl2 in Water Under Aerobic Conditions
B. Liang, M. Dai, J. Chen, Z. Yang, J. Org. Chem., 2005, 70, 391-393.

Sonogashira Coupling Reaction With Diminished Homocoupling
A. Elangovan, Y.-H. Wang, T.-I. Ho, Org. Lett., 2003, 5, 1841-1844.

Carbamoyl-Substituted N-Heterocyclic Carbene Complexes Of Palladium(II): Application To Sonogashira Cross-Coupling Reactions
R. A. Batey, M. Shen, A. J. Lough, Org. Lett., 2002, 1411-1414.

One-Pot Procedure For The Synthesis Of Unsymmetrical Diarylalkynes
R. Severin, J. Reimer, S. Doye, J. Org. Chem., 2010, 75, 3518-3521.

Diastereodivergent Reductive Cross Coupling Of Alkynes Through Tandem Catalysis: Z- And E-Selective Hydroarylation Of Terminal Alkynes
M. K. Armstrong, M. B. Goodstein, G. Lalic, J. Am. Chem. Soc., 2018, 140, 10233-10241.

One-Pot Synthesis Of Diarylalkynes Using Palladium-Catalyzed Sonogashira Reaction And Decarboxylative Coupling Of Sp Carbon And Sp2 Carbon
J. Moon, M. Jeong, H. Nam, J. Ju, J. H. Moon, H. M. Jung, S. Lee, Org. Lett., 2008, 10, 945-948.

Investigation Of An Efficient Palladium-Catalyzed C(sp)-C(sp) Cross-Coupling Reaction Using Phosphine-Olefin Ligand: Application And Mechanistic Aspects
W. Shi, Y. Luo, X. Luo, L. Chao, H. Zhang, J. Wang, A. Lei, J. Am. Chem. Soc., 2008, 130, 14713-14720.

Discovery Of A Novel Palladium Catalyst For The Preparation Of Enynes With A Copper- And Ligand-Free Sonogashira Reaction
X. Mi, M. Huang, Y. Feng, Y. Wu, Synlett, 2012, 23, 1257-1261.

Sonogashira Reaction Using Arylsulfonium Salts As Cross-Coupling Partners
Z.-Y. Tian, S.M. Wang, S.-J. Jia, H.-X. Song, C.-P. Zhang, Org. Lett., 2017, 19, 5454-5457.

Synthesis Of Internal Alkynes Through An Effective Tandem Elimination-Hydrodebromination-Cross-Coupling Of gem-Dibromoalkenes With Halobenzenes
Y. Ji, N. Zhong, Z. Kang, G. Yan, M. Zhao, Synlett, 2018, 29, 209-214.

One-Pot Conversion Of Aldehydes And Aryl Halides To Disubstituted Alkynes Via Tandem Seyferth-Gilbert Homologation/Copper-Free Sonogashira Coupling
A. Sapegin, M. Krasavin, J. Org. Chem., 2019, 84, 8788-8795.

General, Robust, And Stereocomplementary Preparation Of β-Ketoester Enol Tosylates As Cross-Coupling Partners Utilizing TsCl-N-Methylimidazole Agents
H. Nakatsuji, K. Ueno, T. Misaki, Y. Tanabe, Org. Lett., 2008, 10, 2131-2134.

Single-Isomer Tetrasubstituted Olefins From Regioselective And Stereospecific Palladium-Catalyzed Coupling Of β-Chloro-α-iodo-α,β-unsaturated Esters
A. B. Lemay, K. S. Vulic, W. W. Ogilvie, J. Org. Chem., 2006, 71, 3615-3618.

Palladium-Catalyzed Coupling Of Terminal Alkynes With Benzyl Ammonium Salts
S. Xu, Z. Zhang, C. Han, W. Hu, T. Xiao, Y. Yuan, J. Zhao, J. Org. Chem., 2019, 84, 12157-12164.

Palladium-Catalyzed Carbonylative Sonogashira Coupling Of Aryl Bromides Using Near Stoichiometric Carbon Monoxide
K. T. Neumann, S. R. Laursen, A. T. Lindhardt, B. Bang-Andersen, T. Skrydstrup, Org. Lett., 2014, 16, 2216-2219.

Straightforward Novel One-Pot Enaminone And Pyrimidine Syntheses By Coupling-Addition-Cyclocondensation Sequences
A. S. Karpov, T. J. J. Müller, Synthesis, 2003, 2815-2826.

Synthesis Of Isoquinolines And Pyridines By The Palladium- And Copper-Catalyzed Coupling And Cyclization Of Terminal Acetylenes
K. R. Roesch, R. C. Larock, Org. Lett., 1999, 1, 553-556.