
Benzilic Acid Rearrangement
Benzilic Acid Rearrangement

1,2-Diketones Undergo A Rearrangement In The Presence Of Strong Base To Yield α-hydroxycarboxylic Acids. The Best Yields Are Obtained When The Subject Diketones Do Not Have Enolizable Protons.
The Reaction Of A Cyclic Diketone Leads To An Interesting Ring Contraction:

Ketoaldehydes Do Not React In The Same Manner, Where A Hydride Shift Is Preferred (see Cannizzaro Reaction)
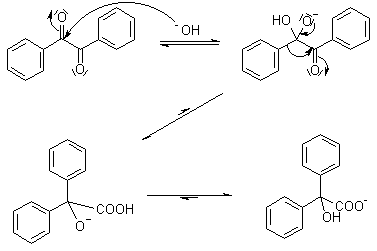
Mechanism Of Benzilic Acid Rearrangement
Recent Literature

Direct Synthesis Of 1,2-Diketones By Catalytic Aerobic Oxidative Decarboxylation Of 1,3-Diketones With Iodine And Base Under Irradiation Of Fluorescent Light
N. Tada, M. Shomura, H. Nakayama, T. Miura, A. Itoh, Synlett, 2010, 1979-1983.